
|
Photophysics and Photochemistry of Transition Metal Compounds |
| Home Research Members Collaborations Publications |

|
||||||||
Two new ethynylbipyridine-linked mono- and bis-tetrathiafulvalene (TTF) derivatives, together with a Ru(II) complex, were synthesized using Sonogashira coupling reactions and characterized by UV/vis spectroscopy and cyclic voltammetry. They display a clear electrochemically amphoteric behavior consisting of two reversible single-electron oxidation waves (typical for TTF derivatives) and one reversible single-electron reduction wave (bpy) and act as donor–acceptor (D–A) systems. Furthermore, for the Ru(II) complex, a quite intense fluorescence originating from the 3MLCT state is observed. | ||||||||
|
||||||||
Three ruthenium(II) polypyridine complexes of general formula [Ru(bpy)3-n(TTF-dppz)n](PF6)2 (n=1-3, bpy=2,2'-bipyridine), with one, two or three redox-active TTF-dppz (4',5'-bis(propylthio)tetrathiafulvenyl[i]dipyrido[3,2-a:2',3'-c]phenazine) ligands, were synthesised and fully characterised. Their electrochemical and photophysical properties are reported together with those of the reference compounds [Ru(bpy)3](PF6)2, [Ru(dppz)3](PF6)2 and [Ru(bpy)2(dppz)](PF6)2 and the free TTF-dppz ligand. All three complexes show intraligand charge-transfer (ILCT) fluorescence of the TTF-dppz ligand. Remarkably, the complex with n=1 exhibits luminescence from the Ru2+dppz metal-to-ligand charge-transfer (3MLCT) state, whereas for the other two complexes, a radiationless pathway via electron transfer from a second TTF-dppz ligand quenches the 3MLCT luminescence. The TTF fragments as electron donors thus induce a ligand-to-ligand charge-separated (LLCS) state of the form TTF-dppz--Ru2+→ -dppz-TTF+. The lifetime of this LLCS state is approximately 2.3 μs, which is four orders of magnitude longer than that of 0.4 ns for the ILCT state, because recombination of charges on two different ligands is substantially slower. | ||||||||
|
 |
|||||||
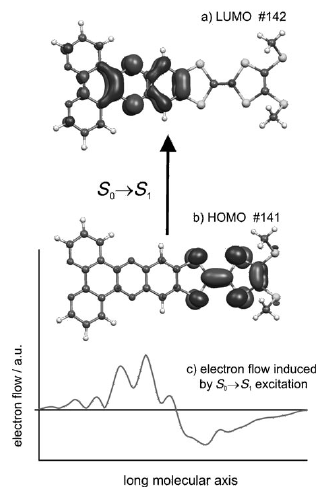
To study the electronic interactions in donor-acceptor (D-A) ensembles, D and A fragments are coupled in a single molecule. Specifically, a tetrathiafulvalene (TTF)-fused dipyrido[3,2-a:2',3'-c]phenazine (dppz) compound having inherent redox centers has been synthesized and structurally characterized. Its electronic absorption, fluorescence emission, photoinduced intramolecular charge transfer, and electrochemical behavior have been investigated. The observed electronic properties are explained on the basis of density functional theory. | ||||||||
|
||||||||
A planar π-conjugated heteroaromatic molecule 1 has been synthesized and fully characterized; it combines two characteristics, a charge-transfer transition originating from its inherent donor–acceptor nature in its neutral state and an intervalence charge-transfer transition in its 12+ mixed-valence state. | ||||||||
|
||||||||
The synthesis of tetrakis(tetrathiafulvalene)-annulated metal-free and metallophthalocyanines 5−8 via the tetramerization of the phthalonitrile derivative 4 is reported. All of them have been fully characterized by electronic absorption spectroscopy, thin-layer cyclic voltammetry, mass spectrometry, and elemental analysis. Their solution electrochemical data show two reversible four-electron oxidation waves, indicating that these fused systems are strong π-electron donors, which give rise to tetra- or octaradical cation species. For the metal-free phthalocyanine 5, additionally a reversible one-electron wave was found in the negative direction arising from the reduction of the macrocycle. Moreover, the tetrathiafulvalene unit acts as an efficient reductive electron-transfer quencher for the phthalocyanine emission, but upon its oxidation, an intense luminescence is switched on. | ||||||||
Download this list in format RIS
 EndNote
EndNote  BibTex
BibTex  PDF XML
PDF XML Last update Friday December 08 2017
